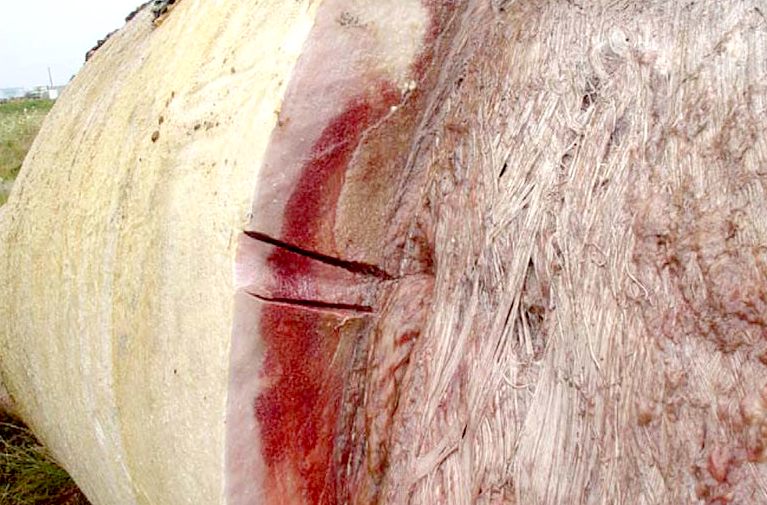
Right
whale carcass being stripped of blubber on land. You can imagine this as
a warm blanket in freezing waters.
Blubber
is what whaling was all about, since this produces the oil. Though
whaling is also about the meat product as a food source. The blubber
(fat) is like a fuel tank. Whales need a lot of fuel because of their
high metabolic rate, that enables them to swim for long periods without
resting. Hence, their engines are always running at high revolutions,
rather than tickover (idling) as when sleeping.
Blubber is the primary fat storage,
specifically for marine mammals that live in water all of their lives. It is particularly important for species that feed and breed in different parts of the ocean. During these periods, the animals metabolize fat. Blubber may
also save energy for marine mammals, such as dolphins, in that it adds buoyancy while swimming.
Blubber has advantages over fur (as in sea otters) in that, though fur retains heat by holding pockets of air, the air expels under pressure (i.e., when the animal dives). Blubber, however, does not compress under pressure. It is effective enough that some whales can dwell in temperatures as low as 40 °F (4 °C). While diving in cold
water, blood vessels covering the blubber constrict and decrease blood flow, thus increasing blubber's efficiency as an insulator.
WHALE OIL
Whale oil is oil obtained from the blubber of whales. Whale oil from the bowhead whale was sometimes known as train oil, which comes from the Dutch word traan ("tear" or "drop").
Sperm oil, a special kind of oil obtained from the head cavities of sperm
whales, differs chemically from ordinary whale oil: it is composed mostly of liquid wax. Its properties and applications differ from those of regular whale oil, and it sells for a higher price.
Early industrial societies used whale oil in oil lamps and to make soap. In the 20th century it was made into margarine. With the commercial development of the petroleum industry and vegetable oils, the use of whale oils declined considerably from its peak in the 19th century into the 20th century. In the 21st century, with most countries having banned whaling, the sale and use of whale oil has practically ceased.
Whale oil was obtained by boiling strips of blubber harvested from whales. The removal is known as "flensing" and the boiling process was called "trying out". The boiling was carried out on land in the case of whales caught close to shore or beached. On longer deep-sea whaling expeditions, the trying-out was done aboard the ship in a furnace known as a trywork and the carcass was then discarded into the water.
Baleen whales were a major source of whale oil. Their oil is exclusively composed of triglycerides, whereas that of toothed whales contains wax esters. The bowhead whale and right whale were considered the ideal whaling targets. They are slow and docile, and they float when killed. They yield plenty of high-quality oil and whalebone, and as a result, they were hunted nearly to extinction.
Whale oil has low viscosity (lower than olive oil), is clear, and varies in color from a bright honey yellow to a dark brown, according to the condition of the blubber from which it has been extracted and the refinement through which it went. It has a strong fishy odor. When hydrogenated, it turns solid and white and its taste and odor change.
The use of whale oil had a steady decline starting in the late 19th century due to the development of superior alternatives, and later, the passing of environmental laws. In 1986, the International Whaling Commission declared a moratorium on commercial whaling, which has all but eliminated the use of whale oil today. The Inuit of North America are granted special whaling rights (justified as being integral to their culture), and they still use whale oil as a food and as lamp oil.
Whale oil was used as a cheap illuminant, though it gave off a strong odor when burnt and was not very popular. It was replaced in the late 19th century by cheaper, more efficient, and longer-lasting kerosene. Burning fluid known as camphine was the dominant replacement for whale oil until the arrival of kerosene.
In the US, whale oil was used in cars as a constituent of automatic transmission fluid until it was banned by the 1973 Endangered Species Act. It was also a major component of tractor hydraulic fluid (like the ubiquitous JDM Type 303 Special Hydraulic Fluid) until its withdrawal in 1974.
In the UK, whale oil was used in toolmaking machinery as a high-quality lubricant.
After the invention of hydrogenation in the early 20th century, whale oil was used to make margarine, a practice that has since been discontinued. Whale oil in margarine has been replaced by vegetable oil.
Whale oil was used to make soap. Until the invention of hydrogenation, it was used only in industrial-grade cleansers, because its foul smell and tendency to discolor made it unsuitable for cosmetic soap.
Whale oil was widely used in the First World War as a preventive measure against trench foot. A British infantry battalion on the Western Front could be expected to use 10 gallons of whale oil a day. The oil was rubbed directly onto bare feet in order to protect them from the effects of immersion.
IN LITERATURE
The pursuit and use of whale oil, along with many other aspects of whaling, are discussed in
Herman Melville's
Moby-Dick. In the novel, the preciousness of the substance to contemporary American society is emphasized when the fictional narrator notes that whale oil is "as rare as the milk of queens." John R. Jewitt, an Englishman who wrote a memoir about his years as a captive of the Nootka people on the Pacific Northwest Coast in 1802–1805, describes how whale oil was used as a condiment with every dish, even strawberries.
Friedrich Ratzel in The History of Mankind (1896), when discussing food materials in Oceania, quoted Captain James Cook's comment in relation to "the Maoris" saying "No Greenlander was ever so sharp set upon train-oil as our friends here, they greedily swallowed the stinking droppings when we were boiling down the fat of dog-fish."

Sperm
whale carcass being stripped of blubber onboard a ship.
TOXICITY OF BLUBBER
In the 21st century, blubber contains man-made polychlorinated biphenyl
(PCBs), carcinogens that damage human nervous, immune, and reproductive systems. The source of PCB concentrations is unknown.
As
toothed whales are high on the food chain, they likely consume large amounts of industrial pollutants
via bioaccumulation.
Baleen whales, by merit of the huge amount of food they consume, are bound to have toxic chemicals stored in their
bodies, since they eat the smaller animals like krill and fish that are
swimming in plastic
polluted seas.
There are high levels of mercury in the blubber of seals of the Canadian arctic.

Gregory
Peck gives an outstanding performance as Captain
Ahab, the obsessed
master of the Pequod, in the 1956
movie: Moby Dick.
A
BIT OF HISTORY
Moby
Dick is the story of a great white
sperm whale that fought back at
whalers who tried to harpoon him. The idea came to Herman Melville after
he spent time on a commercial whaler, where stories abounded of the
sinking of the Essex in 1821 and Mocha
Dick, a giant sperm whale that sank around 20 ships, before being
harpooned in 1838.
Moby
Dick has inspired a great many adaptations, the same basic story
finding its way into the making of four films and two television
adaptations.
In
addition there are many comics and illustrated volumes, adapted from the
original, one of which is the emerging graphic novel version of a large
humpback whale called Kulo Luna.
Kulo
Luna is not as big as the whales depicted in Herman
Melville's Moby
Dick, but she has a diamond encrusted heart of gold, only attacking
whaling ships that present a danger to herself or her friends.

Herman
Melville was the author of what we'd now consider an illegal activity,
the commercial hunting of whales for oil and meat.
Please use our
A-Z INDEX to
navigate this site



